Abstract
INTRODUCTION: Ineffective hematopoiesis in myelodysplastic syndromes (MDS) and chronic myelomonocytic leukemia (CMML) have been associated to upregulated innate immune and inflammatory signals. Spleen tyrosine kinase (SYK) is a non-receptor tyrosine kinase that is highly expressed in hematopoietic cells and mediates signaling through Toll-like receptors and pattern recognition receptors leading to activation of the ERK, AKT, NF-kB and p38 signaling cascades. Given prior data suggesting that inhibition of these signals might be associated with improved hematopoiesis in MDS we hypothesized SYK inhibition might have therapeutic efficacy in lower-risk MDS and CMML.
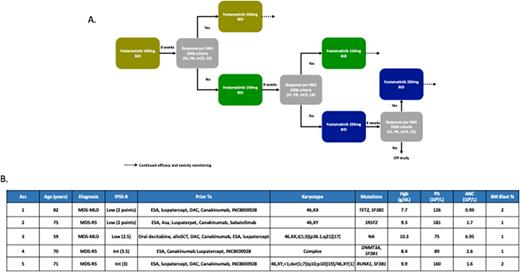
METHODS: To study this we designed an open label phase I clinical trial of fostamatinib, a SYK inhibitor, for patients with lower-risk MDS or CMML (defined as those with a score <3.5 by the revised International Prognostic Scoring System [IPSS-R]) and no response to prior erythroid stimulating agents or hypomethylating agents (HMAs). Patients with MDS with del(5q) must have received prior therapy with lenalidomide and those with MDS with ring sideroblasts must have received luspatercept. Patients had to be transfusion dependent, defined as prior RBC or platelet transfusions within the past 28 days, or have hemoglobin <10g/dL or platelets <75x109/L. Patients with profound neutropenia (ANC <0.5x109/L) where excluded. The study includes an intra-patient dose escalation design by which patients start therapy at a dose of fostamatinib of 100mg po bid on days 1-28 of a 28-day cycle. If no response to therapy, by IWG 2006 response criteria, is observed after 8 weeks of therapy dose will be escalated to 150mg po bid on days 1-28 of a 28-day cycle and then, in the absence of response after an additional 8 weeks of therapy, to 200mg po bid on days 1-28 of a 28 day cycle. Patients achieving no response after the second dose escalation will be discontinued from study (Figure 1a). The primary endpoint of the study is to assess the safety and tolerability of fostamatinib. Secondary objectives included overall response rates, transfusion independence and survival outcomes. The study includes futility and safety stopping rules. This study is registered at clinicaltrials.gov (NCT05030675).
RESULTS: To date, a total of 5 patients have been treated on study as of July 2022 including 3 patients with MDS and ring sideroblasts. Patient characteristics are detailed in Figure 1b. Median age is 71 years (range 59-82). Median number of prior therapies was 5 (range 4-6) including one patient with a prior allogeneic stem-cell transplant. Four (80%) patients had received prior therapy with HMA. Two patients were transfusion dependent of RBC at the time of enrollment with one patient who was eligible based on the presence of thrombocytopenia. At the current data cut off, three patients developed treatment emergent adverse events the main including grade 1-2 diarrhea or constipation. One patient developed COVID-19 infection during the course of therapy and had mild symptomatology. Fostamatinib was not discontinued in this patient. No episodes of expected on-target uncontrolled hypertension or transaminitis have been observed so far. To date, only 4 patients have completed at least 2 cycles of therapy (8 weeks) and are eligible for response, and only 2 patients have dose escalated beyond 100mg po bid: 1 currently at the 150mg po bid dose level, and 1 at the maximum dose of 200mg po bid. With a median follow up of only 2.7 months (95% CI 0-5.6), no responses have been observed. All 5 patients remain on study: 3 (60%) at the 100mg po bid dose, 1 (20%) at 150mg po bid and 1 (20%) at 200mg po bid.
CONCLUSIONS: Preliminary data suggests that treatment with fostamatinib at doses of up to 200mg po bid can be safely administered in patients with lower-risk MDS who have failed available therapies. Enrollment is ongoing to determine the potential efficacy of doses of up to 200mg bid. Additional correlative studies to determine predictors of response are underway.
Disclosures
Kantarjian:KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; NOVA Research: Honoraria; Daiichi-Sankyo: Consultancy, Research Funding; Amgen: Honoraria, Research Funding; ImmunoGen: Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; Takeda: Honoraria. Garcia-Manero:Curis: Honoraria, Research Funding; Aprea: Honoraria; BMS: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Gilead Sciences: Research Funding; Genentech: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Acceleron Pharma: Consultancy; Astex: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal